According to TASS on June 23, Russian materials scientists have discovered that electrodes based on polyaromatic hydrocarbons containing various metal atoms significantly outperform traditional graphite electrodes in capacity and other key performance metrics in many cases for battery production. This was reported by the Skoltech (affiliated with VEB.RF Group) press office.

Skolkovo Institute of Technology Professor Alexander Kvashnin stated: "The capacity of all the studied metal-containing polyaromatic hydrocarbon crystals significantly exceeded the standard values for graphite. For example, the capacities for lithium, sodium, potassium, and rubidium are 1.2 to 1.3 times higher than those of graphite. With the addition of magnesium and calcium, this value is even higher—approximately 2.3 to 2.6 times." His comments were cited by the university's press office.
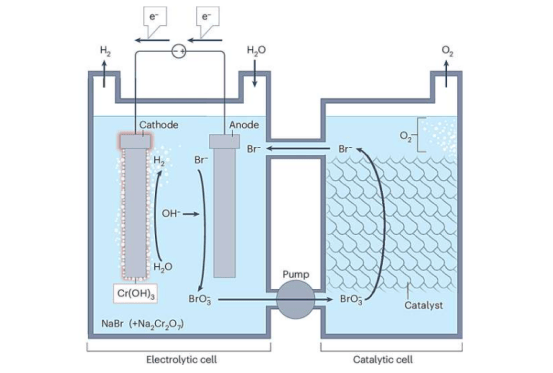
The professors noted that the anodes (negative electrodes) of lithium-ion batteries are typically made from graphite today. While this material has many advantages, its capacity is limited, and production requires heating raw materials to about 3,000 degrees Celsius, increasing the complexity and cost of battery manufacturing.
Senior researcher Ilya Chepkasov from Skolkovo Institute of Technology added: "Over the past few years, scientists have been actively researching anodes made from organic materials (such as polyaromatic hydrocarbons). They are cheaper than graphite and have greater capacity. In our new study, we selected four polyaromatic hydrocarbons—naphthalene, anthracene, tetracene, and pentacene—and embedded various atoms (lithium, sodium, calcium, magnesium, rubidium, and potassium) into their molecular gaps." His remarks were cited by the university's press office.
The scientists' calculations showed that adding these metals to polyaromatic hydrocarbons significantly improves the performance of anodes based on these metals, including capacity—the most important characteristic of lithium batteries. Among them, combinations of tetracene or pentacene with calcium, lithium, or sodium showed the most interesting properties; introducing these atoms into these hydrocarbon materials hardly changes their volume.
The researchers hope that the information gathered will help develop ideal alternatives to graphite anodes, thereby reducing battery production costs, increasing capacity, and improving durability. The scientists concluded that this will pave the way for creating more efficient and cost-effective solutions for electrical energy storage.