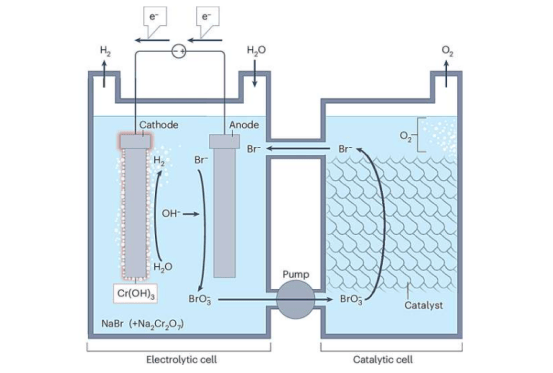
A research team from the University of Chicago Medicine Comprehensive Cancer Center has developed a novel nanoparticle drug delivery system that specifically recognizes cancer cells and releases high concentrations of chemotherapy drugs while minimizing damage to healthy tissue. Published in Cell Reports Medicine, the technology achieves precision dosing by exploiting metabolic differences unique to cancer cells.
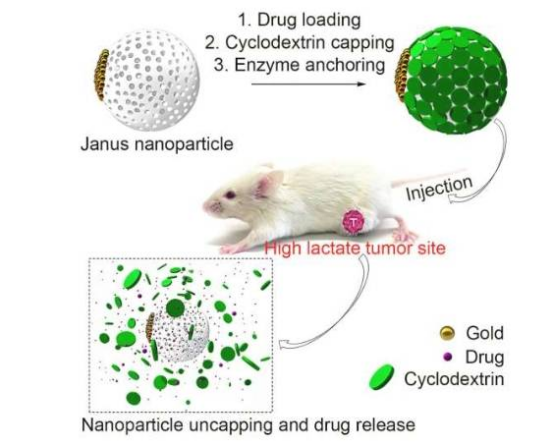
The team designed lactate-responsive nanoparticles based on the Warburg effect. Senior author Dr. Xiaoyang Wu, Associate Professor in the Ben May Department for Cancer Research at the University of Chicago, explained: "Lactate concentrations in the tumor microenvironment can be more than 40 times higher than in normal tissue. Our nanoparticles are engineered to trigger drug release in high-lactate environments while remaining stable in healthy tissue."
Experiments showed that the technology increased drug concentration at tumor sites by 10-fold in mouse models, significantly slowing tumor growth and improving survival rates. Compared to conventional chemotherapy, the system substantially reduced systemic toxic side effects. While current studies focus on doxorubicin, the platform is adaptable to other anticancer drugs and immunotherapies.
Dr. Wu's team has founded Alnair Therapeutics to advance clinical translation. "Scaling up production is the primary challenge right now," he said. "We plan to first optimize the doxorubicin formulation while exploring the platform's potential in treating other cancers."