Researchers at the U.S. Department of Energy's Oak Ridge National Laboratory (ORNL) have utilized the Summit supercomputer to conduct large-scale, high-precision molecular dynamics simulations of the dynamic role of the water-air interface in chemical reactions. This study reveals how water controls chemical reactions through dynamic coupling with reacting molecules, offering new perspectives for developing methods to accelerate interface chemical reactions.
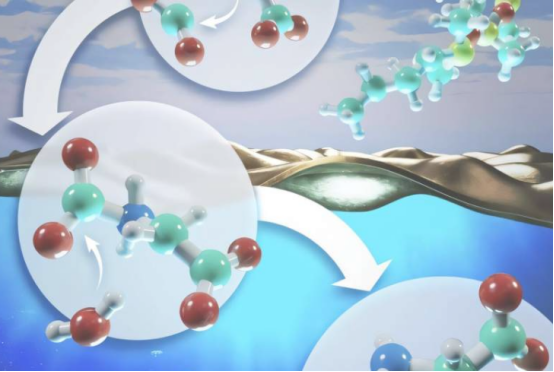
The research team focused on a bimolecular nucleophilic substitution reaction known as SN2, a common reaction mechanism in chemistry, physics, biology, and atmospheric chemistry. For example, in drug synthesis, the SN2 reaction plays a critical role and has been used in the production of ibuprofen.
Vyacheslav Bryantsev, leader of ORNL's Chemical Separations Group, stated: "Our study is the first to answer the question, 'What dynamic role does the air-water interface play in regulating chemical reaction rates?' We found that the overall reaction rate at the air-water interface is faster compared to reactions occurring solely in the bulk water environment."
The simulation results show that chemical reactions between water and air can be accelerated by pulling interacting molecules from the bulk water environment to a position closer to the water surface. This is because reducing the dynamic coupling between water and these molecules allows the chemical reaction to proceed with less interference.
Santanu Roy, a scientist at Oak Ridge National Laboratory, further explained: "The more water molecules are coupled, the greater the hindrance to the reaction. If we can reduce this dynamic coupling, we can increase the reaction rate. Our study shows that by altering the interface environment, we can control this coupling and, consequently, the reaction rate."
The research team used the open-source CP2K code to simulate molecular reaction trajectories on the Summit supercomputer and conducted dynamic analysis of these paths to create an energy distribution map of the process. Roy emphasized: "Without leading computational capabilities, our theories could not be validated or studied. Summit supercomputer provided us with tremendous support."
Additionally, the study simulated the attraction of positively charged surfactant molecules to negatively charged amino acids, confirming a 10% to 15% increase in reaction rate. This finding further elucidates the complex role of water in chemical reaction mechanisms.