A research team led by Professor Karine Le Roch at the University of California, Riverside has made a significant breakthrough in the study of Plasmodium, discovering two key proteins that may serve as new targets for anti-malaria drug development. The study, published in Cell Reports, offers new avenues for treating malaria and other parasitic diseases.
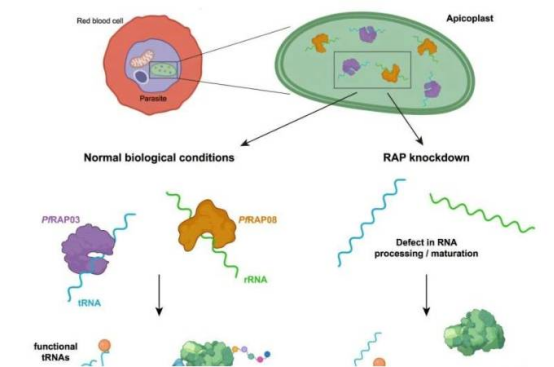
The researchers identified two RAP-family proteins (PfRAP03 and PfRAP08) in the apicoplast of Plasmodium falciparum. These proteins play essential roles by regulating the translation of RNA molecules within this parasite-specific organelle. Experiments showed that inhibiting either protein leads to parasite death.
"This is the first time we've revealed how RAP proteins directly interact with rRNA and tRNA," Professor Le Roch said. Unlike humans, who have only six RAP proteins, Plasmodium possesses more than twenty—a difference that makes them ideal drug targets. The study also found that these mechanisms are widespread in other parasites such as Toxoplasma.
The team is currently working to resolve the 3D structures of these RNA-protein complexes, laying the foundation for future drug design. "Targeting parasite-specific proteins could lead to highly effective, low-toxicity treatments," Le Roch added. The findings are significant not only for malaria but also for combating other apicomplexan parasitic diseases.