A research team led by Professor Feng Pan from the School of Advanced Materials at Peking University Shenzhen Graduate School has achieved a major breakthrough in aqueous battery research, elucidating the key mechanisms of proton storage and transport in aqueous systems. This work provides critical theoretical support for developing safer, faster-charging, and higher-capacity next-generation batteries. The findings, titled "Proton Storage and Transport in Aqueous Batteries," were published in the journal Matter.
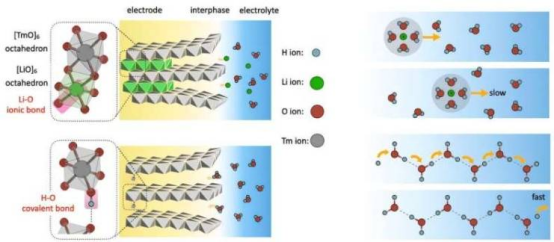
Aqueous batteries are inherently safer than lithium-ion batteries due to their water-based electrolytes, but they have traditionally suffered from lower energy density. Protons, with their low mass and high mobility, are considered highly promising charge carriers. However, their complex chemical behavior has long hindered practical applications. Through in-depth investigation, Professor Pan's team has revealed that protons move via a Grotthuss-like mechanism—hopping between hydrogen bonds—rather than diffusing like metal ions. This discovery enables ultra-fast, "diffusionless" proton transport, laying the foundation for high-performance aqueous batteries.
The study not only addresses the long-standing challenge of simultaneously achieving safety and high performance in energy storage but also proposes three core strategies for optimizing aqueous battery performance through hydrogen-bond network engineering:
Ⅰ.Innovative Electrode Design: Researchers recommend embedding hydrated or anhydrous hydrogen-bond networks within solid-state materials to create well-defined proton transport pathways, thereby enhancing overall battery performance.
Ⅱ.Electrolyte Regulation and Optimization: By precisely tuning acid concentration and anion type in the electrolyte, the team successfully stabilized and enhanced proton conductivity, ensuring efficient battery operation.
Ⅲ.Interface Engineering Improvements: The team found that modifying electrode surfaces—for example, by oxygen plasma treatment to introduce hydroxyl (-OH) and carboxyl (-COOH) groups—creates proton-bridging channels, significantly reducing interfacial charge-transfer resistance and improving reaction kinetics.
These strategies collectively form a unified framework that thoroughly elucidates proton behavior in aqueous systems, paving the way for next-generation proton-based aqueous batteries that combine safety with high performance.
Looking ahead, by rationally designing hydrogen-bond networks, new aqueous batteries are expected to achieve higher energy density, faster charging speeds, and longer cycle life. This breakthrough will strongly advance battery technology applications in grid-scale storage, portable electronics, and electric vehicles, making significant contributions to green manufacturing and sustainable development.