As countries worldwide actively advance transportation decarbonization, hydrogen is emerging as a key contender in areas where battery applications are limited. It shows immense potential, particularly in heavy-duty vehicles, long-haul trucks, ships, and aircraft.
Associate Professor Sun Jianwu from Linköping University (LiU) in Sweden noted that passenger cars can be equipped with batteries, but heavy trucks, ships, or airplanes cannot rely on batteries for energy storage. For these vehicles, clean and renewable energy sources are needed, and hydrogen is an excellent choice.
Sun Jianwu and his team are developing a material that produces hydrogen from water using only sunlight. This "green" hydrogen could power the transportation sector, compensating for the shortcomings of battery systems in these applications.
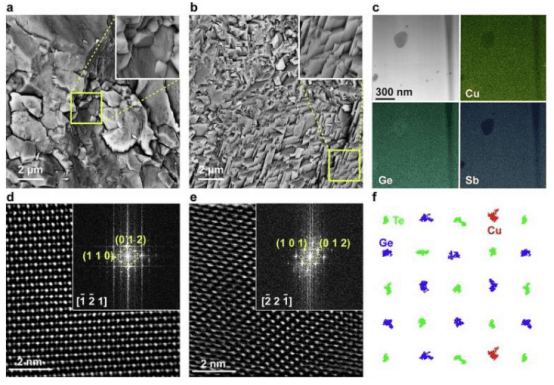
The latest research from the Linköping University team introduces a new material that significantly boosts solar hydrogen production. The researchers constructed a layered structure combining cubic silicon carbide (3C-SiC), cobalt oxide (Co₃O₄), and a nickel hydroxide (Ni(OH)₂) catalyst layer. Professor Sun stated that the research goal was to analyze the role of each layer: "This is a very complex structure, so the focus was on understanding the function of each layer and how they help improve material performance."

When exposed to sunlight, this material generates charges that split water into hydrogen and oxygen. However, a major obstacle in such systems is charge recombination, where positive and negative charges cancel each other out before triggering the reaction. By integrating the three-layer structure, the team achieved better charge separation, reporting an eightfold performance improvement compared to using 3C-SiC alone.
Currently, nearly all hydrogen on the market is "gray hydrogen" produced from fossil fuels, emitting up to 10 tons of CO₂ per ton of hydrogen, undermining its status as a clean fuel. In contrast, "green" hydrogen uses renewable electricity for water splitting, but even this method often relies on energy beyond direct sunlight. The Sun Jianwu team aims to eliminate this dependency, driving the reaction solely with solar energy to reduce costs and eliminate carbon emissions during the process.
Despite the promising outlook, the technology is not yet commercialized. Most materials currently used for solar water splitting have efficiencies of only 1–3%, while scaling "green" hydrogen requires material efficiency of at least 10%. Sun Jianwu believes this target is achievable, estimating that his team may need about 5 to 10 years to develop a material reaching this efficiency.
Achieving this benchmark would mark a turning point for the hydrogen economy, enabling low-cost, carbon-free hydrogen production at a scale sufficient to support industrial and transportation uses. If successful, the layered material developed by Professor Sun's team could become a cornerstone of clean fuel infrastructure in the coming decades. The research results have been published in the Journal of the American Chemical Society.