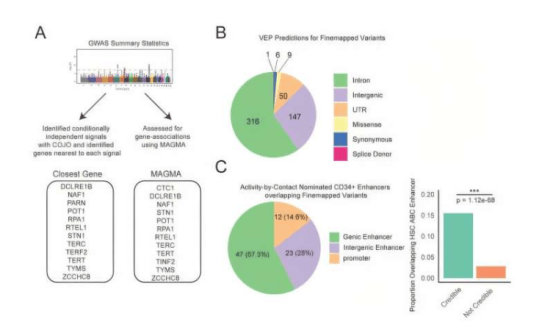
In recent years, energy engineers have been actively engaged in developing more sustainable power generation and electricity storage technologies. Among them, electrolyzers serve as a key device that uses electricity generated from renewable sources such as photovoltaics and wind turbines to split water (H₂O) into hydrogen (H₂) and oxygen (O₂) through electrolysis. The hydrogen produced by electrolyzers can then be used in fuel cells—devices that convert the chemical energy of hydrogen directly into electricity without combustion—providing power for heavy-duty vehicles such as trucks, buses, and forklifts, as well as backup power for facilities like hospitals and data centers.
Among various electrolyzer designs, many recently developed electrolyzers employ proton exchange membranes (PEM) to facilitate the splitting of water into hydrogen. The proton exchange membrane selectively allows protons (H⁺) to pass while blocking gases. Research has shown that, compared to the currently common alkaline electrolyzers, PEM electrolyzers produce hydrogen of higher purity. However, PEM electrolyzers also have clear drawbacks: they are more expensive and require ultra-pure water, as impurities (such as cations, anions, and other contaminants) cause the equipment to degrade rapidly over time.
To address this issue, researchers from Tianjin University and other institutions have recently developed a strategy to improve PEM electrolyzer catalysts, enabling them to break down impure water. Details of this strategy were published in a paper in Nature Energy.
Paper authors Luguang Wang, Yuting Yang, and colleagues noted: "PEM electrolyzers typically use ultra-pure water as feedstock because trace contaminants in the feed water, especially cationic impurities, can cause failure. Developing PEM electrolyzers capable of tolerating low-purity water can minimize water pretreatment, reduce maintenance costs, and extend system lifetime."
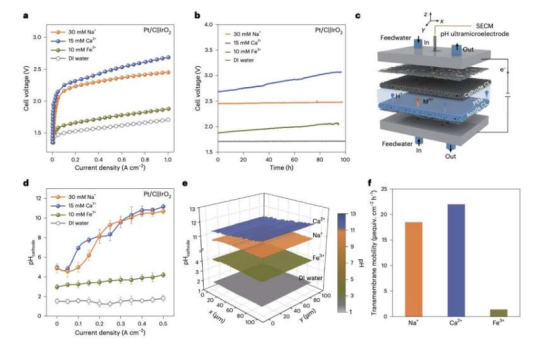
Against this backdrop, the research team developed a PEM electrolyzer with microenvironment pH regulation. It can operate stably for over 3,000 hours in impure ("ap") water at a current density of 1.0Acm⁻² while maintaining performance comparable to state-of-the-art PEM electrolyzers using pure water.
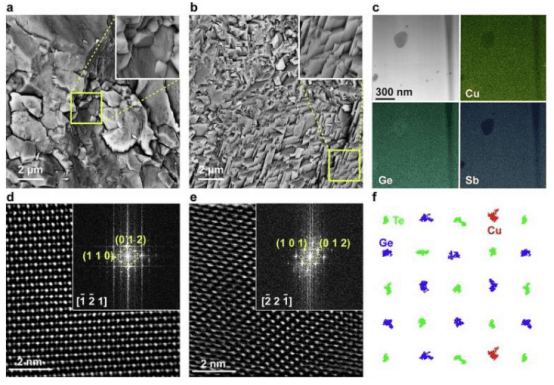
To evaluate the potential of this strategy, Luguang Wang, Yuting Yang, and their colleagues added the Brønsted acidic oxide MoO₃₋ₓ to cathodes made of platinum on carbon (Pt/C). They found that when this cathode was integrated as a catalyst into a proton exchange membrane (PEM) electrolyzer, performance was enhanced, enabling reliable hydrogen production from pure water without rapid degradation over time.
The team also used techniques combining pH microelectrodes and scanning electrochemical microscopy to monitor local pH conditions in situ within the PEM electrolyzer. They discovered that Brønsted acidic oxides can lower the local pH. Therefore, they introduced the Brønsted acidic oxide MoO₃₋ₓ onto the Pt/C cathode to create a strongly acidic microenvironment, thereby enhancing the kinetics of hydrogen production, suppressing deposition/precipitation on the cathode, and inhibiting membrane degradation.
This research opens new possibilities for the design of PEM electrolyzers, helping reduce reliance on ultra-pure water and making them easier to deploy in real-world environments. In the future, other energy engineers can build on this team's findings to develop more PEM electrolyzers capable of reliably splitting impure water into hydrogen.