A research team from the École Polytechnique Fédérale de Lausanne (EPFL) and Kyoto University has achieved a major breakthrough by mixing two simple chemicals to synthesize a stable hydrogen-rich liquid, providing a new solution for safe and efficient hydrogen storage at room temperature. The related research findings have been published in the journal Advanced Materials.
Hydrogen is regarded as a future clean fuel, but storage technology has long been a key bottleneck restricting its large-scale application. Traditional hydrogen-rich materials often require storage under extreme conditions (such as high pressure or low temperature) and suffer from low release efficiency and multiple byproducts. For example, ammonia borane can store large amounts of hydrogen but requires heating for release and generates impurities.
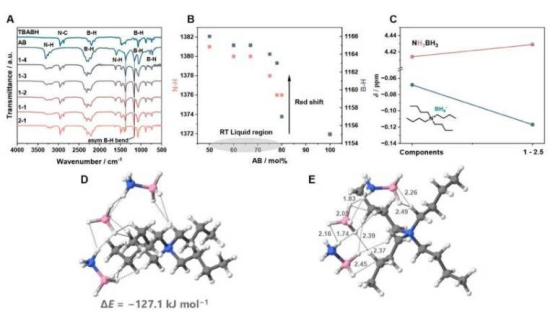
In this study, Professor Andreas Züttel from EPFL and Professor Satoshi Horike from Kyoto University developed for the first time a deep eutectic solvent (DES) based on hydrides—a transparent, stable hydrogen-rich liquid. The liquid remains in liquid state at room temperature, with a maximum hydrogen content of 6.9% (by weight), not only surpassing the U.S. Department of Energy's 2025 hydrogen storage target but also breaking the limitation of traditional DES not containing hydrides, opening a new path for liquid hydrogen storage.
The research team physically mixed ammonia borane with tetrabutylammonium borohydride and found that when ammonia borane accounts for 50% to 80%, the mixture forms a stable amorphous liquid with a glass transition temperature as low as -50°C, far below room temperature. Spectroscopic analysis confirmed strong hydrogen bonding between molecules, disrupting the original solid structure and maintaining fluidity even at extremely low temperatures.
Experiments showed that the liquid releases pure hydrogen simply by heating to 60°C, with significantly higher efficiency than traditional hydrogen-rich solids (which require over 200°C), and some components are reusable, reducing costs and waste.
The stability of the new DES (storable for weeks in dry conditions) and its low-density characteristics make it an ideal alternative to high-pressure tanks or cryogenic liquids. Industry can use this to achieve safe and convenient room-temperature hydrogen transportation and storage, accelerating the implementation in fields such as hydrogen fuel cell vehicles and distributed energy.
Additionally, this technology provides new ideas for customized liquid development and may be applied in the future to chemical production, green energy storage, and other fields, promoting innovation across the entire hydrogen energy industry chain.
The research team pointed out that this discovery solves the triple challenges of "safety, efficiency, and cost" in hydrogen storage, laying the foundation for the hydrogen economy to move from laboratory to daily life. With material optimization and scaled production advancing, the new DES is expected to become a key technology carrier in the energy transition.