Researchers have successfully developed an aqueous organic redox flow battery (AORFB) with stable performance, showing no significant capacity degradation over 220 charge-discharge cycles.
The team led by Professor He Gang from Xi'an Jiaotong University is dedicated to addressing ongoing challenges in stability and cost for energy storage systems through novel electrolyte design. In the new study, the researchers note that flow batteries are considered ideal candidates for large-scale energy storage devices due to their high energy density, long lifespan, and excellent safety performance.
The core of this development is a modified naphthalene diimide (NDI) derivative used as the battery's anolyte. Since AORFBs operate with earth-abundant components in water-based solutions, the battery is regarded as suitable for large-scale energy storage applications.
NDI derivatives are candidate materials for the anolyte (negative electrolyte) because each molecule can store two electrons, contributing to higher battery energy density. However, their practical application has been limited by certain performance issues. The side chains and imide rings of NDI molecules are susceptible to nucleophilic attack by hydroxide ions in aqueous electrolytes, leading to molecular decomposition; radical-induced molecular aggregation increases electrolyte viscosity, affecting battery operation. Although previous improvements enhanced the solubility of NDI materials, molecular stability and cycle durability still need enhancement.
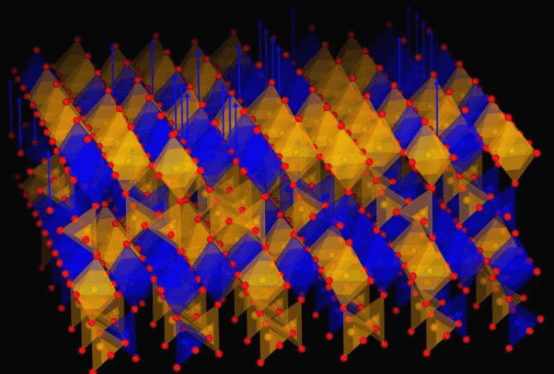
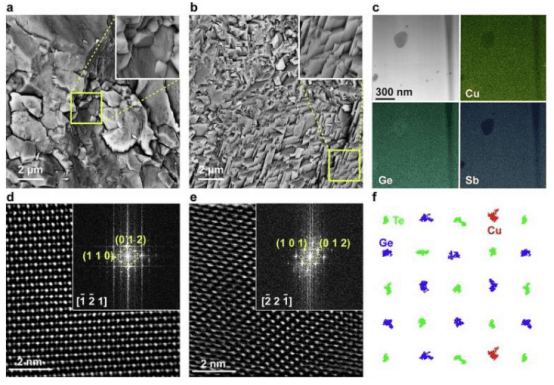
To address these problems, the researchers synthesized zwitterion-modified NDI derivatives using an atmospheric pressure method. After introducing zwitterions with both positive and negative charges, electrostatic repulsion between NDI molecules results in a parallel staggered stacking pattern, with an angle of 42.8° between adjacent molecules and a stacking distance of 3.45Å.
This structure produces multiple effects. On one hand, the four charged centers on the zwitterionic NDI molecule ((CBu)2NDI) increase its solubility to 1.49M; on the other hand, this arrangement enhances the aromaticity of the molecule in its reduced state, helping it maintain stability during electron transfer processes.